Our perception of the world is shaped by our past experiences and expectations, made possible by the incredible flexibility of brain circuits. Still, the neural changes that occur to support adaptive behavior in a dynamically changing environment are not well understood.
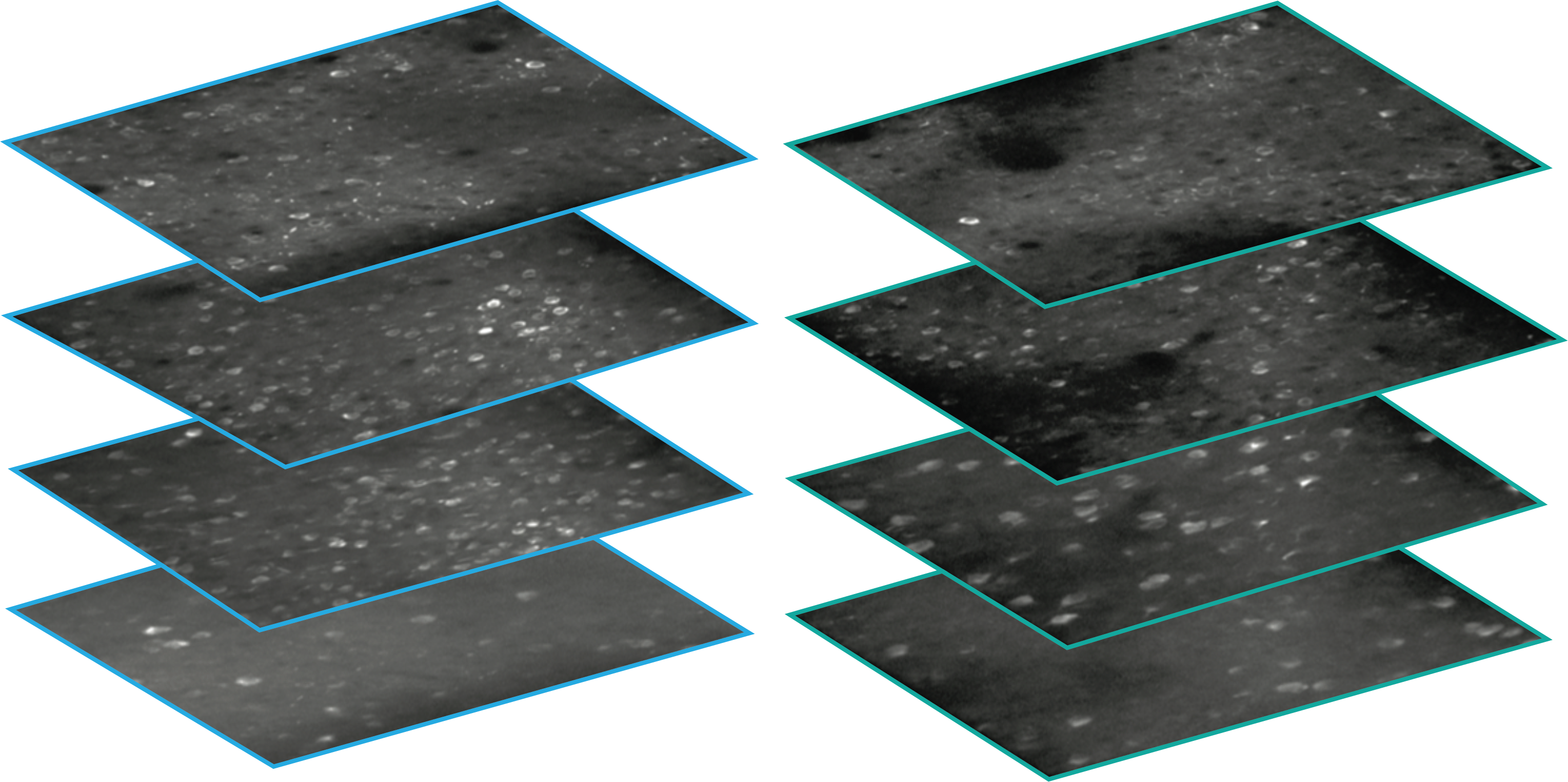
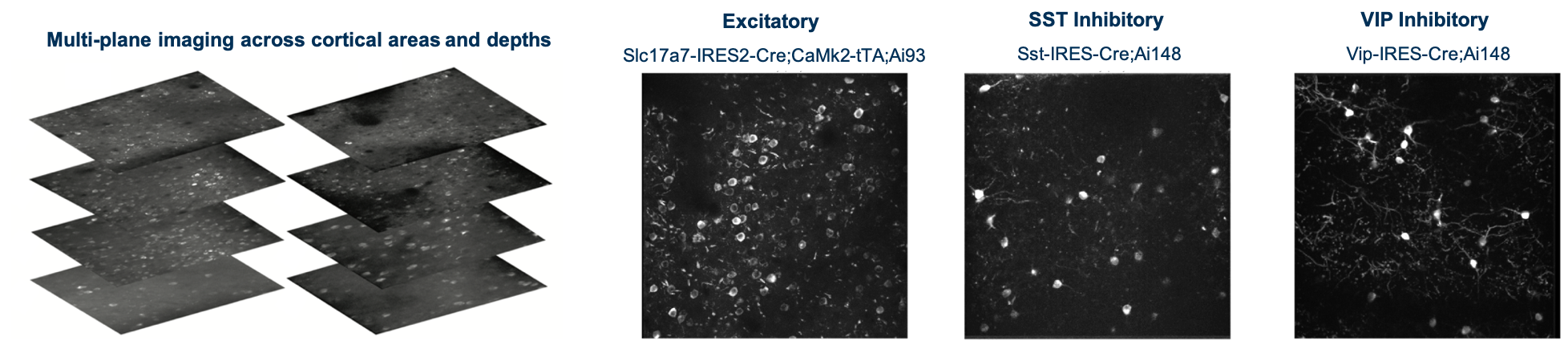
Using single- and multi-plane 2-photon calcium imaging in the visual cortex of transgenic mice expressing the calcium indicator GCaMP6f in populations of excitatory and inhibitory neurons, we have recorded neural activity during performance of a visual change detection task from 34,619 neurons in 551 in vivo imaging sessions. A key aspect of the experimental design is the repeated imaging of the same populations of neurons across multiple days, allowing analysis of single cell changes across behavioral and sensory conditions, including task engagement and stimulus novelty.
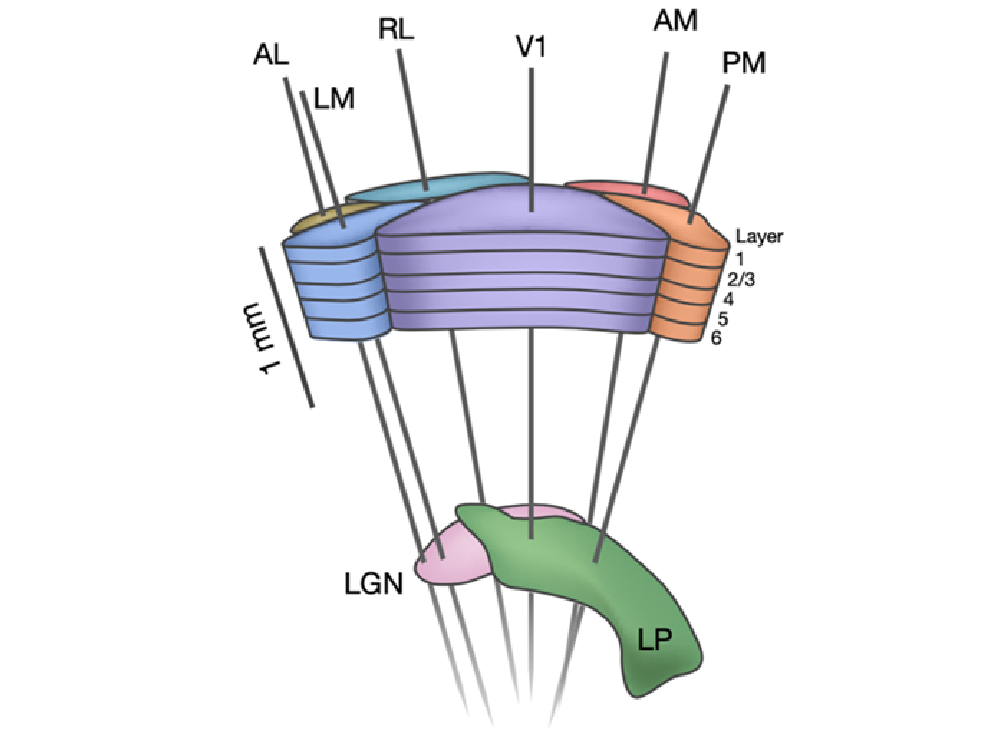
We used Neuropixels probes to measure spiking activity of neuronal populations distributed across multiple visual cortical regions, in addition to subcortical structures such as the thalamus, hippocampus and midbrain, while mice performed the change detection task.
Overall, this dataset includes ~200,000 recorded neurons (units) from 153 experimental sessions. The simultaneous recording of activity across multiple visual areas permits analysis of inter-regional interactions and signal flow during visually guided behavior. In addition, each experimental session includes a passive stimulus replay block that allows investigation of task-dependent modulation sensory and behavioral coding.
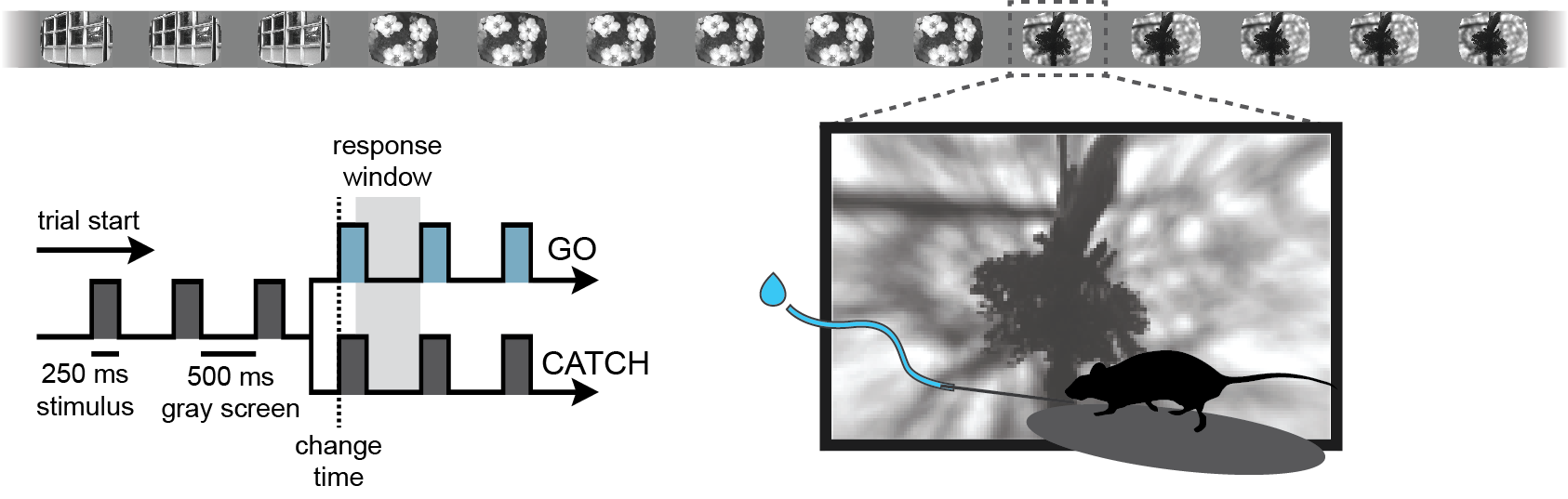
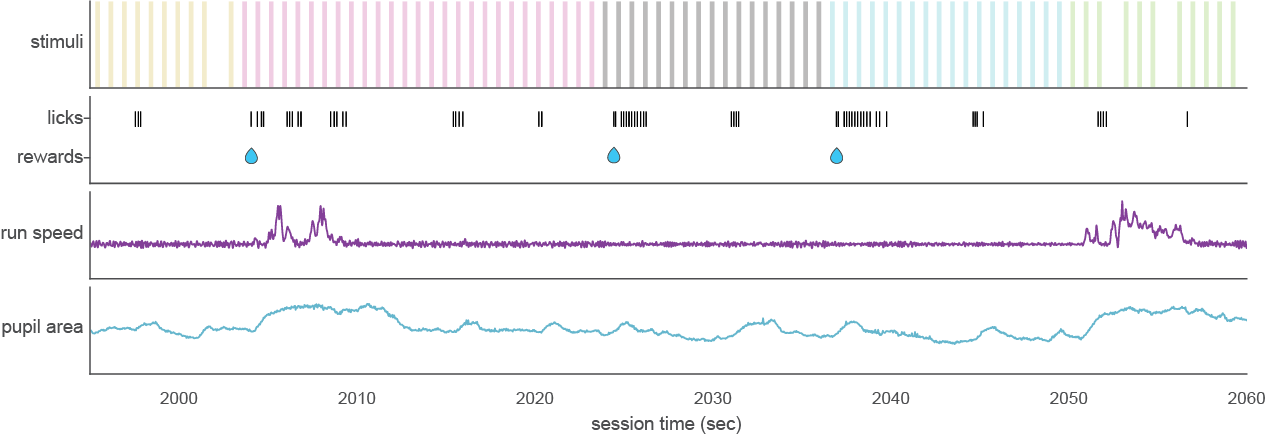
The Visual Behavior Optical Physiology and Visual Behavior Neuropixels projects are built upon a change detection behavioral task. Briefly, in this go/no-go task, mice are shown a continuous series of briefly presented visual images and they earn water rewards by correctly reporting when the identity of the image changes (diagrammed below).
This dataset includes single- and multi-plane 2-photon calcium imaging recordings from the visual cortex of transgenic mice expressing the calcium indicator GCaMP6f in populations of excitatory (Slc17a7-IRES2-Cre;Camk2a-tTA;Ai93(TITL-GCaMP6)) and inhibitory (Vip-IRES-Cre;Ai148(TIT2L-GC6f-ICL-tTA2) & Sst-IRES-Cre;Ai148(TIT2L-GC6f-ICL-tTA2)) neurons.
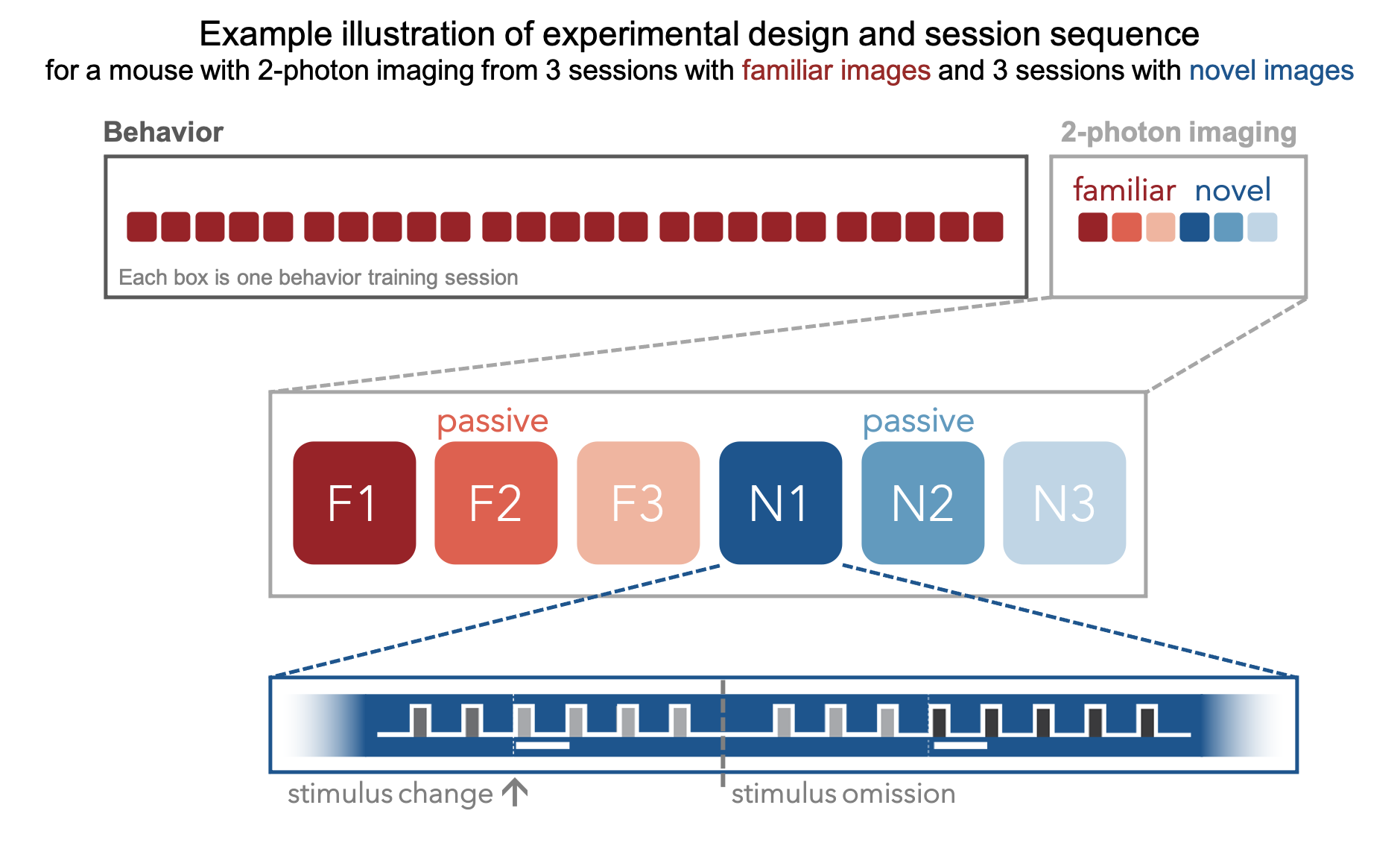
During the imaging portion of the experiment, mice perform the task with the set of natural images they viewed during training (indicated in red in the schematic below), as well as a novel set of images that they have not seen before (indicated in blue). This allows evaluation of the impact of novelty on neural coding for stimulus and behavioral information. Mice also undergo passive viewing sessions during the imaging phase, during which they observe the stimulus in open loop mode and are unable to earn water rewards. Lastly, during imaging sessions only, the expected cadence of flashed images was disrupted by omitting 5% of non-change image flashes.
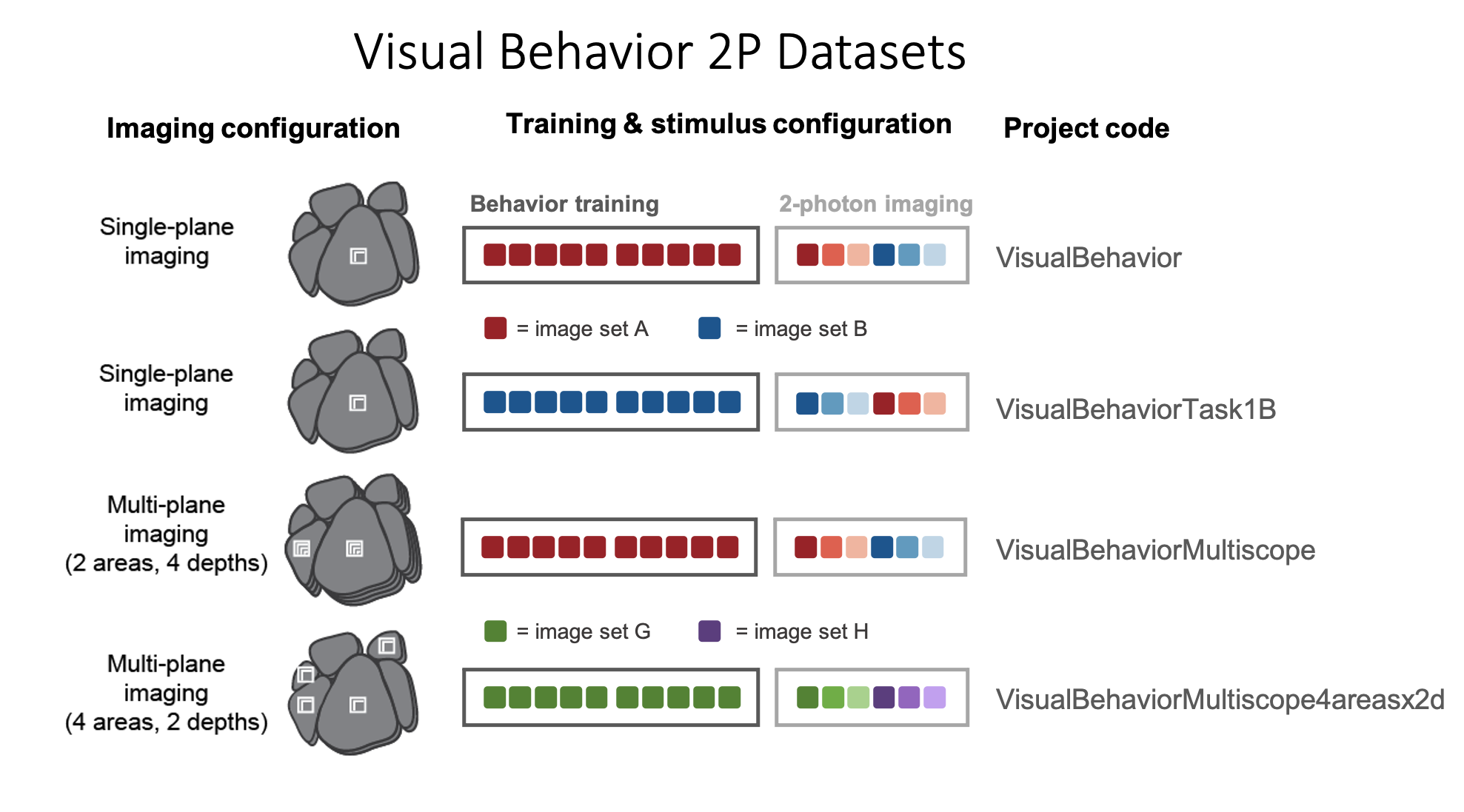
To ensure that any observed effects of stimulus novelty are due to lack of experience with those images, rather than properties of the specific images that were used, we trained a subset of mice with the opposite stimulus configuration such that the training set for these mice was the image set that was novel for a different group of mice (compare dataset variants VisualBehavior and VisualBehaviorTask1B in the schematic below).
A key aspect of the experimental design involved the repeated targeting of populations of neurons across multiple days, allowing analysis of single cell changes across behavioral and sensory conditions, including familiar and novel stimuli, engaged behavior, and passive viewing.
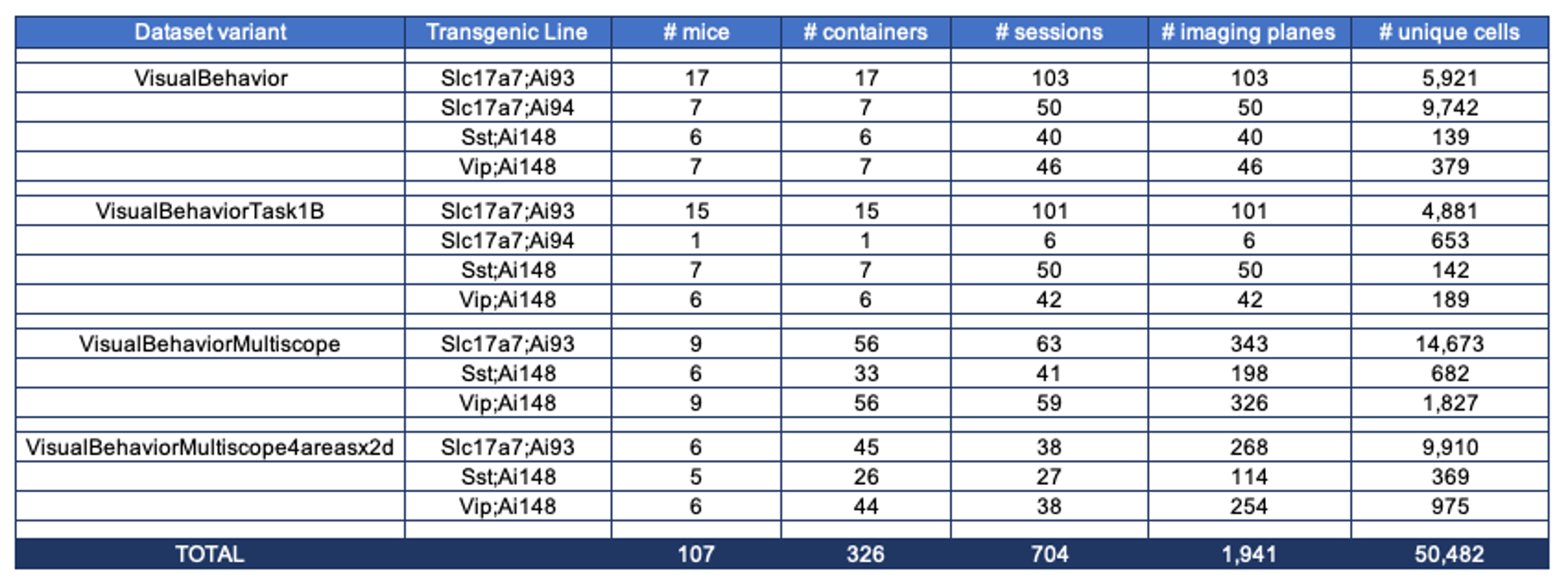
The table below describes the numbers of mice, sessions, imaging planes, and unique recorded neurons for each transgenic line for each of the dataset variants:
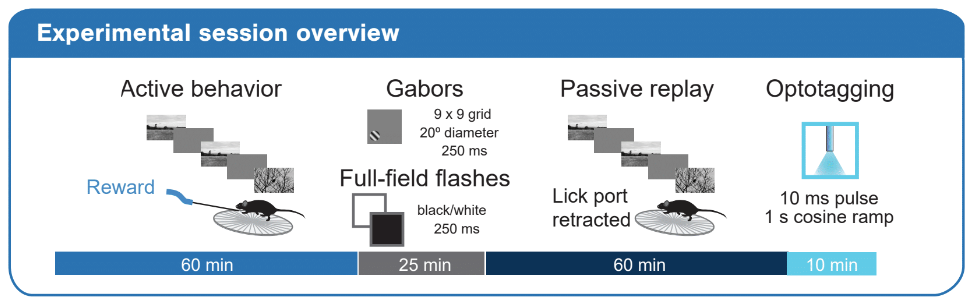
Every experimental session consisted of four major stimulus epochs as diagrammed below: 1) an active behavior epoch during which the mouse performed the change detection task, 2) a receptive field characterization epoch during which we presented gabor stimuli and full-field flashes, 3) a passive replay epoch during which we replayed the same stimulus frame-for-frame as the mouse encountered during active behavior, but now with the lick spout removed and 4) an optotagging epoch during which we stimulated the surface of the brain with blue light to activate ChR2-expressing cortical interneurons.
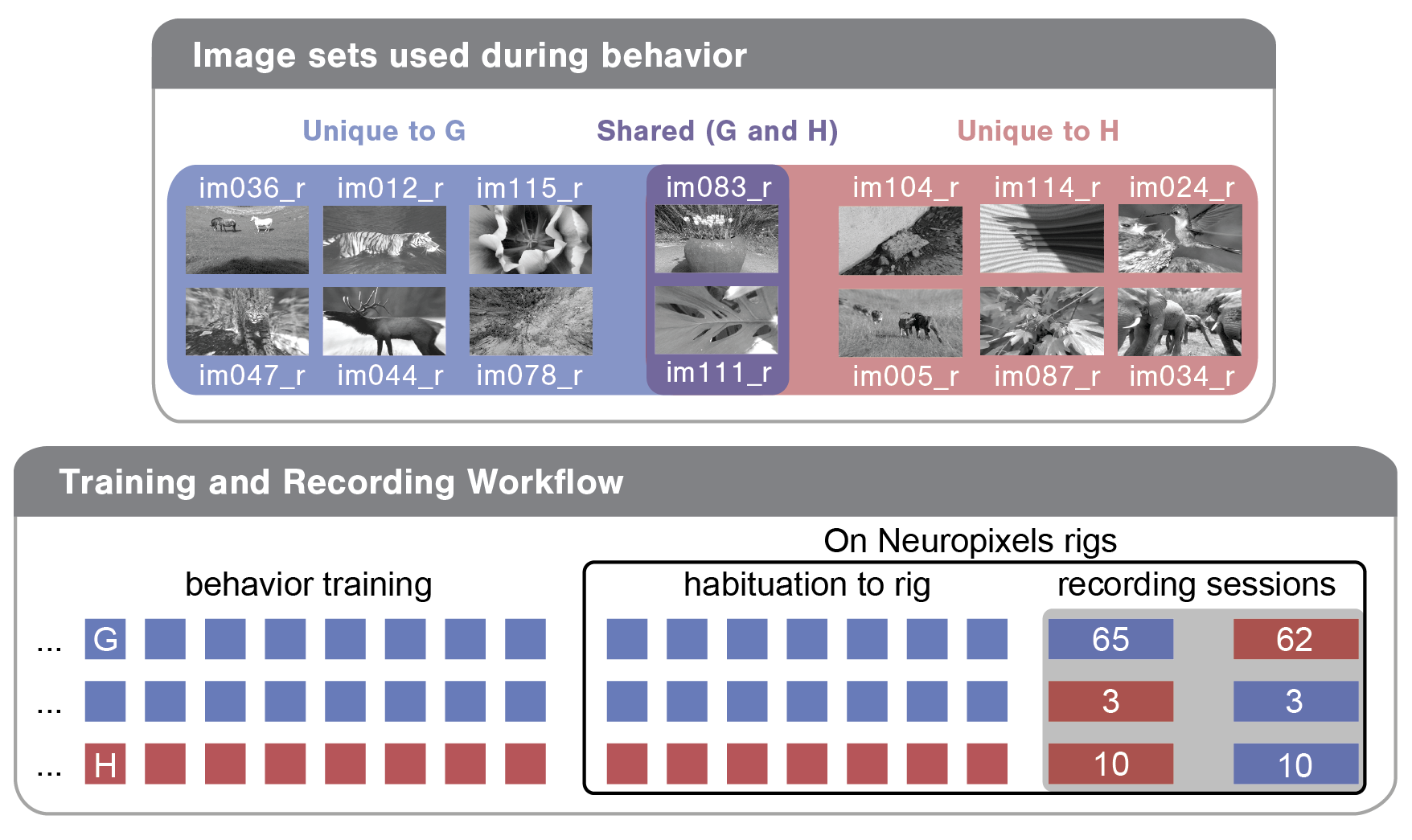
To allow analysis of stimulus novelty on neural responses, two different images sets were used in the recording sessions: G and H (diagrammed below). Both image sets comprised 8 natural images. Two images were shared across the two image sets (purple in diagram), enabling within session analysis of novelty effects. Mice took one of the following three trajectories through training and the two days of recording:
1) Train on G; see G on the first recording day; see H on the second recording day
2) Train on G; see H on the first recording day; see G on the second recording day
3) Train on H; see H on the first recording day; see G on the second recording day
The numbers in the Training and Recording Workflow bubble below give the total recording sessions of each type in the dataset.